Dr. Sandra Lee of NJ Labs discusses how to prepare for tick season and what to do if you’ve been bit, via 360 Magazine


Dr. Sandra Lee of NJ Labs discusses how to prepare for tick season and what to do if you’ve been bit, via 360 Magazine

Health is important, and having a good diet is necessary, but with a million “experts” out there, it’s hard to know what to take seriously. This is why to trust scientists like Nicole Avena, PhD, Associate Professor of Neuroscience at Mount Sinai School of Medicine and Visiting Professor of Health Psychology and Princeton University. She […]

By: Skyler Johnson Learning how to play an instrument can help with the development of the human brain, according to scientist, inventor and Northwestern University professor Dr. Nina Kraus. She outlines this research in her new book, Of Sound Mind: How Our Brain Constructs a Meaningful World. I had the opportunity to interview Dr. Kraus about […]
The Henry Ford’s Invention Convention Michigan honored student inventors for their outstanding inventions and problem-solving solutions during a virtual awards ceremony. Based on this year’s entries, 15 student inventors from across the state will be participating in the Raytheon Invention Convention U.S. Nationals, powered by The Henry Ford, taking place virtually on June 24, 2021. […]
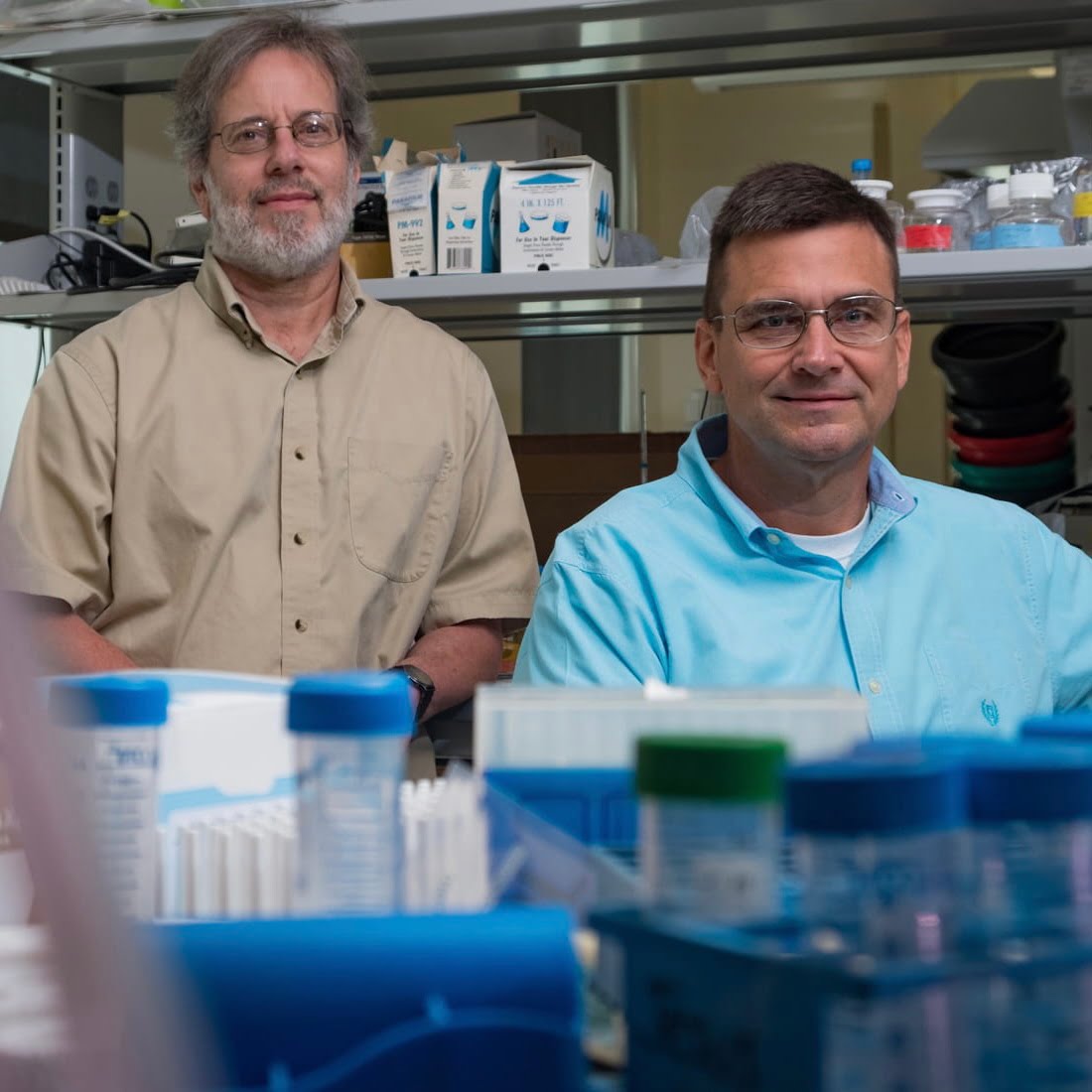
Michael Stern and James McNew (Photo by Jeff Fitlow/Rice University) Study: Early, late stages of degenerative diseases are distinct Two-phase theory applies to diseases like Alzheimer’s, Parkinson’s, muscle atrophy Rice University biochemists Michael Stern and James McNew have studied how neurodegeneration kills cells. They’ve conducted countless experiments over more than a decade, and they’ve summarized […]

A new treatment option for lung fibrosis is being developed by Purdue University scientists. Lung fibrosis has been a concern for COVID-19 patients. People with idiopathic pulmonary fibrosis (IPF) have a life expectancy of fewer than five years. Fibrotic diseases cause organ failure that leads to about 45% of all deaths in the United States. […]

Rice University, MD Anderson research points toward better personalized therapy A combination of drugs that affect mitochondria — the power plants inside cells — may become the best weapons yet to fight acute myeloid leukemia, according to Rice University researchers. A study led by Rice bioscientist Natasha Kirienko and postdoctoral researcher Svetlana Panina found that […]

As the headlines warn of a world seemingly taking steps backward, behavioral scientist Dr. Kristen Lee shares a new psychology of thinking to move you forward with a new mindset and patterns of behaviors that inspire connection, collaboration, proactivity, and creativity. Based on twenty years of clinical practice and neuroscientific research, Dr. Kristen Lee teaches […]